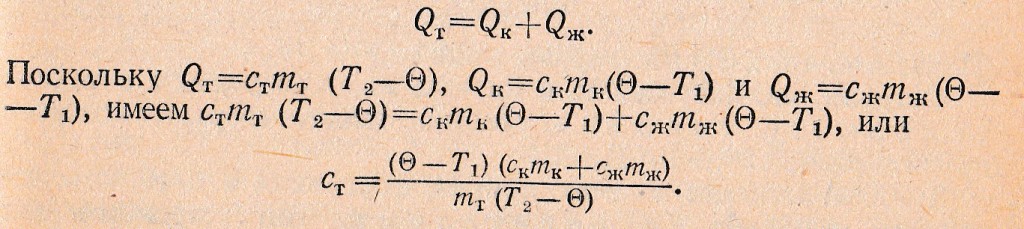Remember, in the absence of mechanical work changes the internal energy of the body estimate the amount of heat Q. Since a pure heat transfer any other kinds of changes in the internal energy is not, on the basis of the energy conservation law can be argued, in this case, how much warmth will give some of the body, столько же приобретут другие. On this basis compiled the equation of thermal balance, which made all the calculations.
So, when the heat exchange amount of the heat amount, given all the bodies, the internal energy decreases, equal to the sum of amounts of heat, all of the bodies, the internal energy increases:
Heat transfer occurs until, while the temperature of the bodies is not equal. The General temperature, which is obtained after the heat exchange, denote Ɵ (Greek. theta).
For example, make an equation of thermal balance, which is used in determining the specific heat capacity of a substance using a calorimeter. Approximately can be considered, in this case, the heat transfer involves three bodies: the calorimeter, liquid and the body, specific heat of the substances which determine the. This body is preheated to a certain temperature T2 and lowered into the calorimeter with the liquid, the temperature T1. After some time in the calorimeter establishes a common final temperature tel Ɵ. It could be argued, in the process of heat exchange the body gave the quantity of heat QT, while the calorimeter and the liquid, respectively QTo and QW. So:
Substituting into the right side of the last formula numeric values, derived from experience, calculate the specific heat of the substance of the body.